Fluorescence Can Save Lives
For those of us lucky enough to have been to an indoor mini-golf course, adventurous enough to walk through a dimly lit haunted house, or attend a late-night rave, you may have some idea of how bright and visually amazing fluorescent paint can look. Besides being used to visually entice people with logic defying brightness though, fluorescent pigments have found a well-deserved home in the underappreciated, but never overlooked area of personal safety. Going from merely interesting to life saving attention grabbers, fluorescent yellows and oranges have become so common place that if asked to think of a school bus or a traffic cone, there is almost no doubt that the first colors thought of are yellow and orange respectively. To gain a better understanding of how exactly fluorescent pigments work and how colors in general are perceived by the human eye we need to establish a brief background around both topics. Truly understanding these concepts is best achieved through everyday examples so don’t worry about being too bogged down by the chemistry and physics of fluorescent optics, there will be plenty of colorful diagrams and simple to understand analogies.
A definition of color is the optical effect produced by any portion or portions of the visible spectrum striking the human eye. The important part to note is that when humans are exposed to light from the sun, they are being exposed to an entire ‘spectrum’ of electromagnetic energy, but our eyes can only perceive a small portion of that spectrum that we refer to as ‘visible light’. The diagram below helps illustrate this idea while also showing that frequency, wavelength, and energy are all related. These concepts are related because light is energy that moves through space in the form of waves, meaning that a certain wavelength of electromagnetic radiation (light), will always have a corresponding known energy.
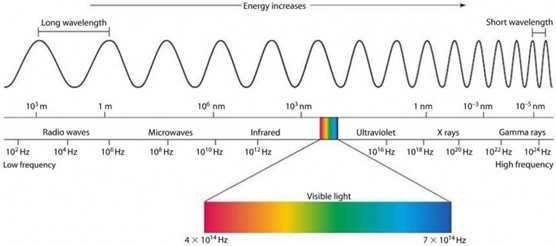
The physical equation that relates energy to wavelength and frequency is as follows: E = hv = hc/λ. E= energy h= Planck’s constant (6.626 x 10-34JHz-1) v= frequency c= speed of light (2.998 x 108 m/s) λ= wavelength The below diagram illustrates the various colors and their corresponding wavelengths and is helpful to see that, for example violet light at 400 nm has a shorter wavelength than red light at 665nm. Due to having a shorter wavelength, through the above equation this means the violet light will also have a higher energy.
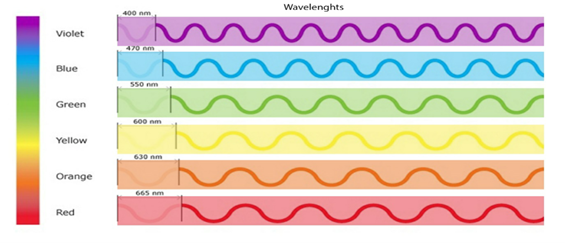
This type of relationship is known as an inverse relationship, where in one variable (wavelength) decreases, the other variable (energy) increases. How our human anatomy handles certain amounts of energy is very important in understanding how color is perceived and to showcase the importance that energy has, you simply need think about the range just beyond violet, Ultraviolet or UV. Ultraviolet rays from the sun are known to be harmful to unprotected human skin and can even cause health issues such as cancer if exposure is long enough. This is because the ultraviolet rays are a shorter wavelength (too short for humans to see) and therefore a higher energy, a high enough amount of energy to physically damage human skin cells. Ouch! So, we have established that light is energy and that it moves through space in the form of waves, and a small portion of these waves are what humans perceive to be the colors of the rainbow. Now here is where things get interesting, because while there are several physics equations, we can use to understand light on a quantum level, there are a lot more factors regarding the anatomy and physiology of the human eye that go into our brains perceiving that color.
The eye is an incredibly specialized and intricate part of human anatomy as any ophthalmologist can tell you. Without getting too complicated and discussing every single part, lets instead focus on photoreceptor cells, and the rods and cones that make them up.
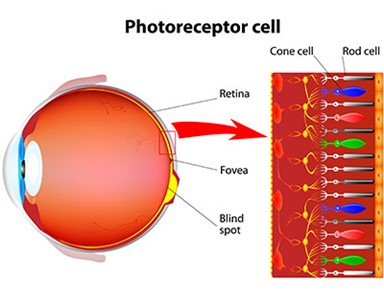 As one may notice from the colored diagram above, there are several cone cells illustrated, but some are red, others green and finally there are even a few that are blue. This may seem random, but it is done intentionally to help relay the concept of trichromatic vision in humans.
As one may notice from the colored diagram above, there are several cone cells illustrated, but some are red, others green and finally there are even a few that are blue. This may seem random, but it is done intentionally to help relay the concept of trichromatic vision in humans.
Trichromatic theory indicates that we (and other primates) can receive 3 types of colors (red, green, and blue) and that the cones vary the ratio of neural activity, similar to how a projection television works by using only primary colors of varying intensity to create every color in-between (Nickerson, 2022). This ratio of each color to the other determines the exact color that we see, and these ratios are determined by the three different cone receptors.
Luckily these cone receptors have some easy to remember names since they are named after the wavelength range of light they are most sensitive to. Short wavelength cone receptors or S-cones (blue), medium wavelength or M-cones (green) and long wavelength or L-cones (red). You can see a chart below that illustrates the sensitivity of the cone receptors across the visible light range (Nickerson, 2022). Something worth noting is that there is a significant overlap between the ‘Green’ receptor and ‘Red’ receptor at around 555nm. This will become more important when discussing which colors are most easily perceived by the human eye in various light conditions.
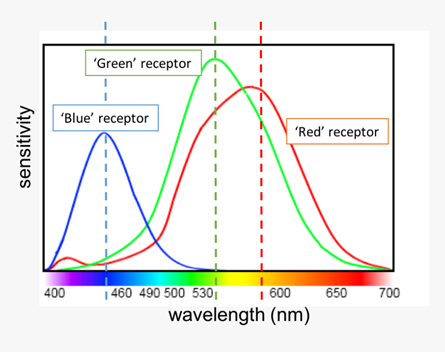
Two interesting things about the cone receptors is that the S-cones make up only 5% of the total number of cones in the retina and they are less sensitive to light than M- and L- cones. Being less sensitive to light means that they are not as important to how humans perceive brightness but are still essential in perceiving color (Parker, 2019) Just like the overlap between the green and red receptors at 555nm mentioned earlier, these pieces of information will be crucial in understanding which colors are most perceptible to humans and under what conditions.
Surrounded by skyscrapers, highways and sprawling city landscapes may be commonplace now, but our ancestors had more of a natural background of forests and foothills in their daily lives. As anyone who has walked through a nature reserve or even local park will tell you, the dominating color in these settings is almost always green. This should be no surprise then that the most easily visible color to the human eye during daylight conditions is green at 555nm. There have even been studies done relating the exposure of ‘Greenness’ to a longer life, showcasing just how important this color is to humans and their well-being (https://ehp.niehs.nih.gov/doi/10.1289/ehp.1510363#tab2). This study focused on the impact on female participants, but the premise still stands and shows just how crucial something as simple as the color green can be to human life, both daily and long-term.
With green being the most visible color to humans, it seems odd that most of the safety colors people use are often yellow or orange, so why is this the case? Going back to our simple diagram of the eye, you can see the three cone receptors we discussed, but there are also the Rod cells. These Rod cells are cylindrically shaped cells that are most sensitive to light and dark changes, shape and movement and contain only one type of light sensitive pigment (as opposed to the three cone types). What this means is that in a dimly lit room humans use primarily Rod cells to view their environment, and have trouble distinguishing colors, while in bright daylight they use their cones. Essentially, in conditions of low light, instead of green being the most easily visible color at a distance, yellow is.
Taxis, school buses, even some firetrucks and emergency response vehicles will have a large amount of yellow on them to be more visible at night, when people are most likely to have trouble seeing color in general.

Now that we have established how light works, how the human eye perceives this light to create colors and which colors are brightest under certain lighting conditions, we can move onto tackling the concept of fluorescent pigment compared to conventional pigment. With conventional pigment, and colored objects in general, the color you observe is the wavelength of light that the object is reflecting into your eye, due to it absorbing all other wavelengths of visible light. A ripe banana as we know appears yellow, but that is only the reflected color, meaning that the banana is absorbing every other color of visible light, except for yellow. May seem a little counterintuitive at first, but this distinction between absorbance and reflectance is helpful for understanding exactly what makes fluorescent materials so visually stunning.
Fluorescence is defined as the phenomenon in which light energy of a relatively short wavelength is converted into visible light energy of a longer wavelength. This shift in wavelength is referred to as a Stokes shift and an example diagram depicting it can be seen below.
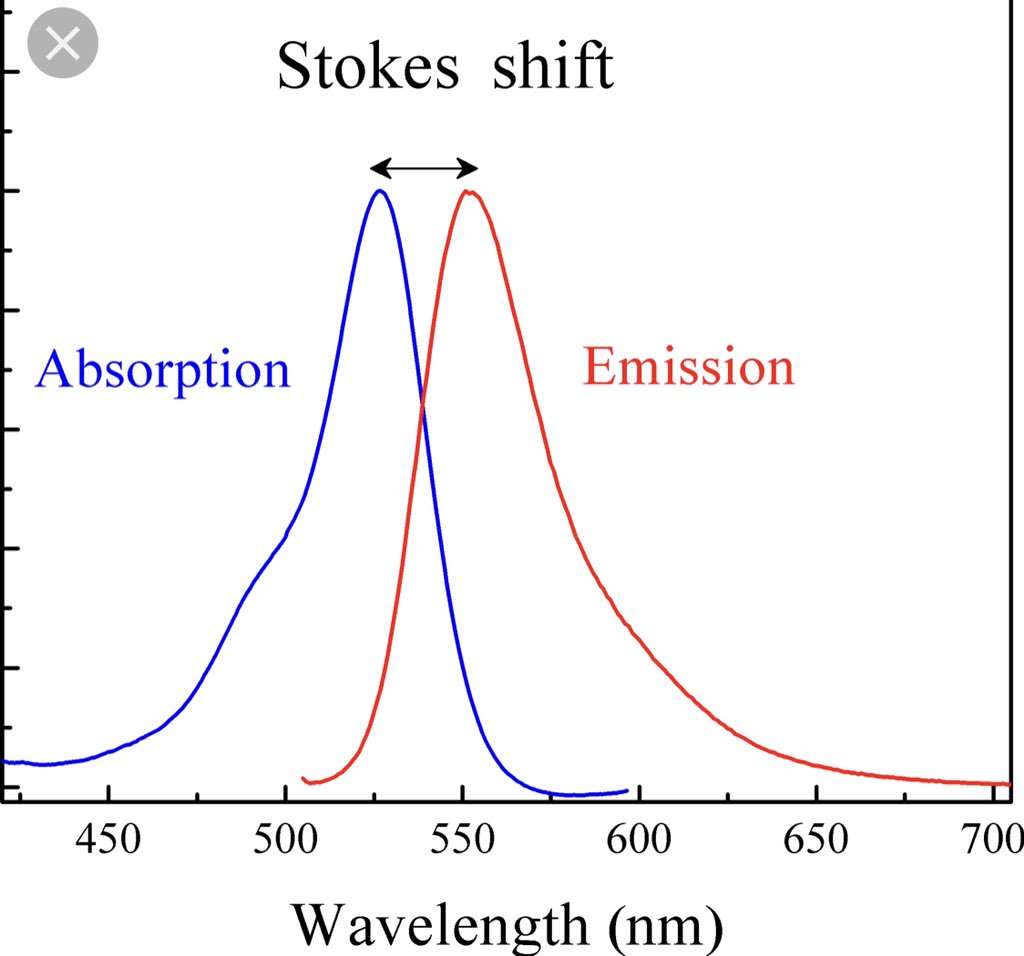 As previously mentioned, all colored materials are absorbing and reflecting various wavelengths of light and the color we observe is a result of the wavelengths that aren’t being absorbed by the object instead being reflected towards our eye. This same process happens with fluorescent materials but with the added energy of converting some of that absorbed light (shorter wavelength) into re-emitted light at a different (longer) wavelength that matches the color being reflected and therefore increases its brightness.
As previously mentioned, all colored materials are absorbing and reflecting various wavelengths of light and the color we observe is a result of the wavelengths that aren’t being absorbed by the object instead being reflected towards our eye. This same process happens with fluorescent materials but with the added energy of converting some of that absorbed light (shorter wavelength) into re-emitted light at a different (longer) wavelength that matches the color being reflected and therefore increases its brightness.
Placing a conventional red and a fluorescent red next to each other will have both samples reflecting red light towards your eye, but because of how the physics of fluorescence works, the fluorescent pigment sample will appear visually brighter. This goes from somewhat helpful during daytime, to almost essential at night.
A specially made time lapse video can be found below that shows firsthand just how much brighter fluorescent pigments are compared to conventional pigments of the same color. In the video you will see a small painted metal panel that has three equal sections of paint, with DayGlo GT-17N on the far left, a conventional yellow in the middle and a DayGlo’s Endure Light-fast Yellow on the far right.
Link: https://youtu.be/LPU25unt9xc
Both DayGlo products on the far right and left can clearly be seen much longer and more clearly than the middle section containing the conventional yellow pigment, illustrating just how much of an impact choosing a fluorescent material over conventional can have regarding personal safety. The far-right section appears brighter than the far-left despite both being fluorescent pigments, due to it being a specially modified DayGlo product designed to have increased brightness of color in addition to being more light-fast. For anyone who doesn’t know what ‘light-fastness’ is, it is a colorant such as a dye or pigments ability to resist fading when exposed to light. This means that not only can you create products with much higher visibility in low light conditions, but they will perform for even longer without fading.
Besides being brighter in low-light settings, another reason fluorescent materials in general are used for safety materials is due to them also having increased visibility in weather conditions like fog, haze, or heavy rain due to the physics of how fluorescence functions. Longer wavelengths of light simply do not have the energy necessary to penetrate fog or haze and the result is a general darkening of all conventional colors during these conditions (Smith, n.d.). This loss of penetration power occurs because the excess water vapor in the air diffuses the light, making any colored surface appear darker because there isn’t enough light reflecting off the surface to reach the eye with the same intensity as it normally would. Since fluorescent materials can convert shorter wavelengths of light that do possess the energy necessary to penetrate through the increased water vapor in the air, and convert them to the longer wavelengths, they are able to reinforce their color and remain more visible.
Next time you see a school bus or a crossing guard wearing a bright orange safety vest, think about all the work that goes into making these materials and improving their functionality to help save lives. At DayGlo we are a part of that effort and strive to make longer lasting, higher durability products through constant research, development and testing because sometimes the difference between life and death is a thin yellow line.
APA Style References:
Nickerson, C. (2022, April 29). The Trichromatic Theory of Color Vision. Simply Psychology. www.simplypsychologoy.org/what-is-the-trichromatic-theory-of-color-vision.html Parker,D. (2019). Color Perception. SAFE Publications. Young, T. (1802). II. The Bakerian Lecture. On the theory of light and colors. Philosophical transactions of the Royal Society of London, (92), 12-48 Smith, H. J. (n.d.). Daylight Flourescent Color- The color That Shouts. Onlinepubs.trb.org. Retrieved May 27, 2022, from https://onlinepubs.trb.org/Onlinepubs/trcircular/229/229-003.pdf
